IEC 60601-1-2:2014 + A1:2020 (New Amendment A1)
In 2020, a new amendment A1 was published that made some changes to IEC 60601-1-2:2014.
The crucial changes:
- Conducted disturbances (conducted emissions) shall be performed at min. and max. RATED voltage,
- NEW TEST – Immunity to proximity magnetic fields (9 kHz–13,56 MHz) – the manufacturer shall consider whether the medical device is sensitive or operates near magnetic sources. The requirements arise from the RFID requirements based on AIM 7351731
- New risk analysis requirements related to the proximity magnetic fields,
- Conducted disturbances (conducted immunity) shall be performed for all signal lines longer than 1 m,
- New normative references (standards) leading to the different test setups (the use of CMAD is mandatory for radiated emission measurements LINK).
Additional testing is required primarily, but not exclusively, for body-worn and hand-held devices under the following conditions:
- The medical device contains magnetically sensitive components or circuitry,
- The separation distance of 15 cm is not maintained,
- The risk of exposure and performance degradation could result in an unacceptable risk.
The EN version has been published recently and will become mandatory in the EU in three years. However, The FDA recognized the latest IEC 60601-1-2:2014 + A1:2020 standard at the end of 2020.
The latest standards with correct nomenclature:
- IEC 60601-1-2:2014 + A1:2020
- EN 60601-1-2:2015 + A1:2021
We hereby announce that we test according to the latest medical standard IEC 60601-1-2:2014 + A1:2020 for which we have IECEE CB accreditation. We can perform immunity tests against magnetic proximity fields caused by induction cooking appliances, RFID readers, wireless power charging systems, etc.
Please do not hesitate to contact us if you have any additional questions – sales@siq.si.
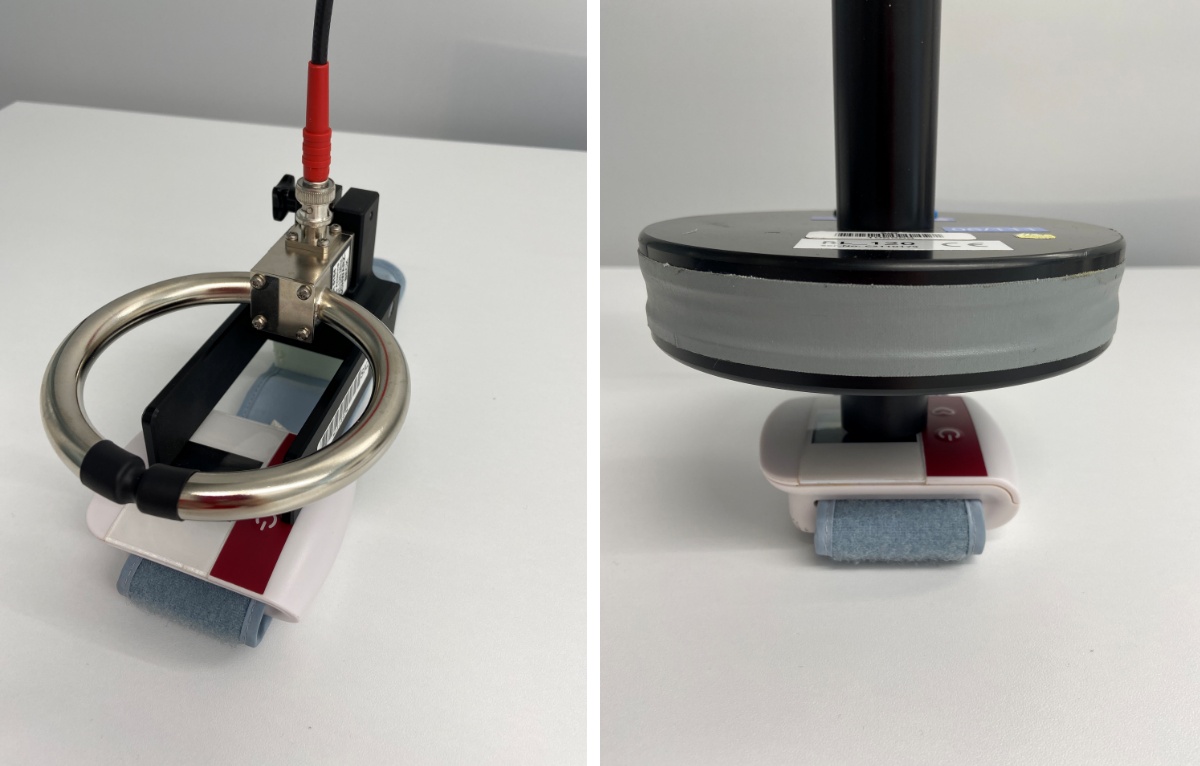
New test – Immunity to proximity magnetic fields