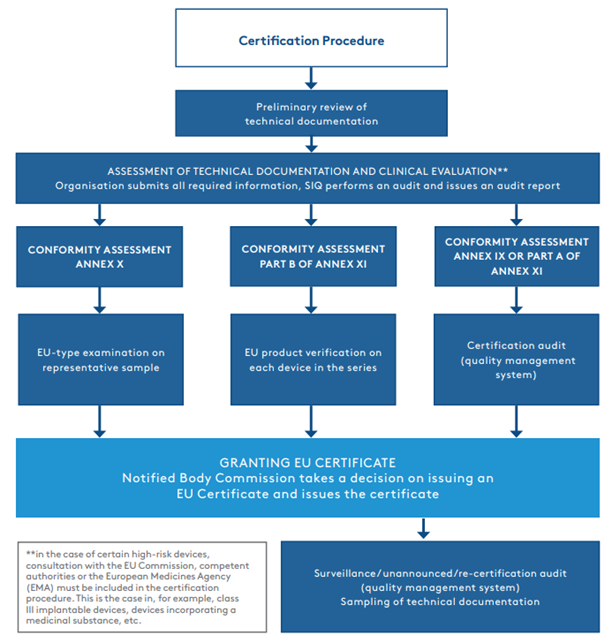
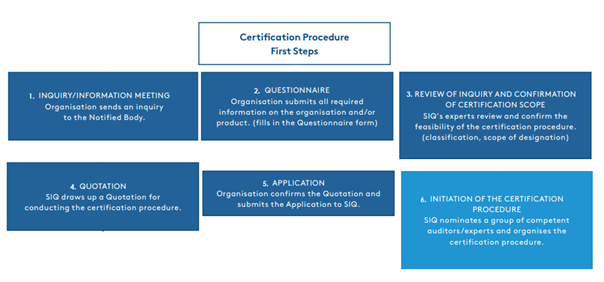
Certification procedure according to Regulation (EU) 2017/745
The first part of the procedure is the assessment of the technical documentation and the clinical evaluation of the device. Once the assessment of the technical documentation and the clinical evaluation has been completed and the documentation is in compliance with the MDR, the certification procedure continues according to the chosen conformity assessment procedure.
Most often, manufacturers decide on a conformity assessment procedure set out in Annex IX or Part A of Annex XI, which involves an audit of the established quality management system. In such cases, the certification procedure continues with a certification audit, which may be combined with an audit according to the ISO 13485:2016 standard. EU certificate is valid for 5 years. Once a year, we check the compliance with the quality management system with surveillance audits.