Training programmes
Our complete solutions in the field of medical devices include expert medical device compliance training programmes – interpretation of standards and regulations.
You need to update your browser to view the website. If you still encounter issues after updating, we suggest you to use a different browser.
Do you manufacture, install, service or sell medical devices? Can you prove that your medical device is safe and that it complies with all the relevant legislative requirements? By implementing and certifying the quality management system according to the ISO 13485 standard, you will prove to the buyers and users of the medical devices that you meet both their requirements and the requirements of national legislation. The ISO 13485 standard is an international standard and therefore it is recognized worldwide.
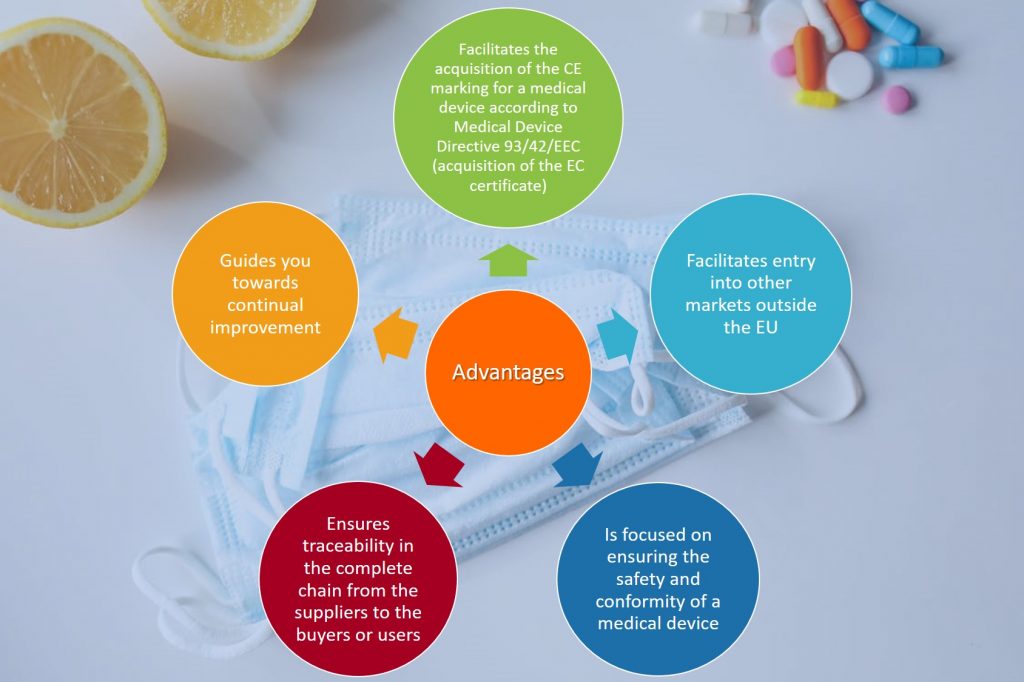
With the certificate you will demonstrate to buyers, business partners and other interested parties that you meet the requirements of the ISO 13485 and therefore:
The certification is a procedure applied by an independent institution (SIQ) to provide written evidence that a product, process or service complies with the requirements of the reference standard. This is confirmed with the ISO 13485 certificate, which is used as evidence of compliance with the requirements. The certification procedure consists of a certification audit – stage 1 and 2. After the annual granting of the certificate, surveillance audits are conducted on specific parts of the system to check the functioning of the system, and a re-assessment audit is conducted every three years. The ISO 13485 certificate is valid as long as you can prove that you still comply with the requirements of the ISO 13485 standard by means of annual audits. The audit according to the ISO 13485 standard can be combined with the medical device certification according to Medical Device Regulation MDR(EU) 2017/745. In this case, the technical file of the medical device and additional requirements for quality management system of the Medical Device Regulation are also assessed in the procedure.
Because the same management principles are applied to various aspects of an organization’s operations, it is becoming increasingly common to speak of integrated management systems. The ISO 13485 standard is based on the ISO 9001 standard.
The already established quality management system according to ISO 9001 can be updated with the additional requirements of the ISO 13485 standard, such as: working environment requirements, risk assessment, planning and development, the provision of traceability and reporting, the cleanliness and sterilization of products, installation and servicing of products, the validation of processes and responsibility of subcontractors.
Most commonly, the ISO 13485 standard is combined with an audit according to Medical Device Regulation MDR (EU) 2017/745.
Our complete solutions in the field of medical devices include expert medical device compliance training programmes – interpretation of standards and regulations.
