All news
SIQ among the world’s top CB certificate issuers
The IECEE CB scheme is an international system for mutual acceptance of test reports and certificates dealing with the safety of electrical and electronic components, equipment, and products. It is a multilateral agreement among participating coun...
Find out more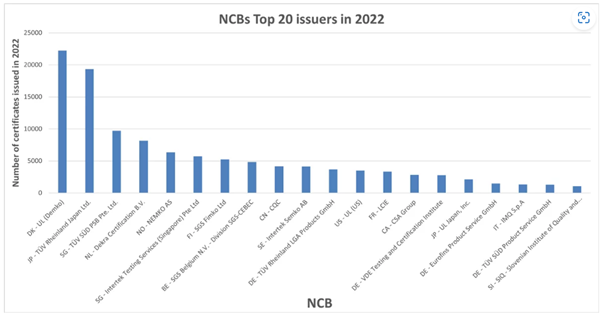
A new edition of IEC 61851-1 (edition 4) is currently in preparation
IEC 61851-1 applies to EV supply equipment for charging electric road vehicles, with a rated supply voltage of up to 1 000 V AC or up to 1 500 V DC and a rated output voltage of up to 1 000 V AC or up to 1 500 V DC. Electric road vehicles (EV) cov...
Find out more
IEC 62368-1 4th edition published
Edition 4 of IEC 62368-1:2023 was published on May 26, 2023. At the moment, it is still not known what the outcome of the HAS consultant assessment in the EU will be. Due to the huge backlog of projects, the date of the HAS assessment is not de...
Find out more
EPSMA Management Committee meeting
On May 25, 2023, SIQ participated in EPSMA (European Power Supply Manufacturing Association) Management Committee meeting. Among other topics, members also discussed the carbon footprint of power supply products. EPSMA Technical Committee will dis...
Find out more
American and Canadian requirements for maximum permissible exposure (MPE)
SIQ is in the accreditation process by Slovenian Accreditation (SA) to start testing against American and Canadian requirements for maximum permissible exposure (MPE). SIQ follows the development of wireless communications and wireless energy t...
Find out more